Device companies spend a tremendous amount of time, money and energy developing and implementing biocompatibility testing programs. Pacific BioLabs has developed the BioPT (Biocompatibility Planning Tool) to guide you through the basic concepts of device testing and to help manufacturers select testing procedures to comply with current regulatory requirements.
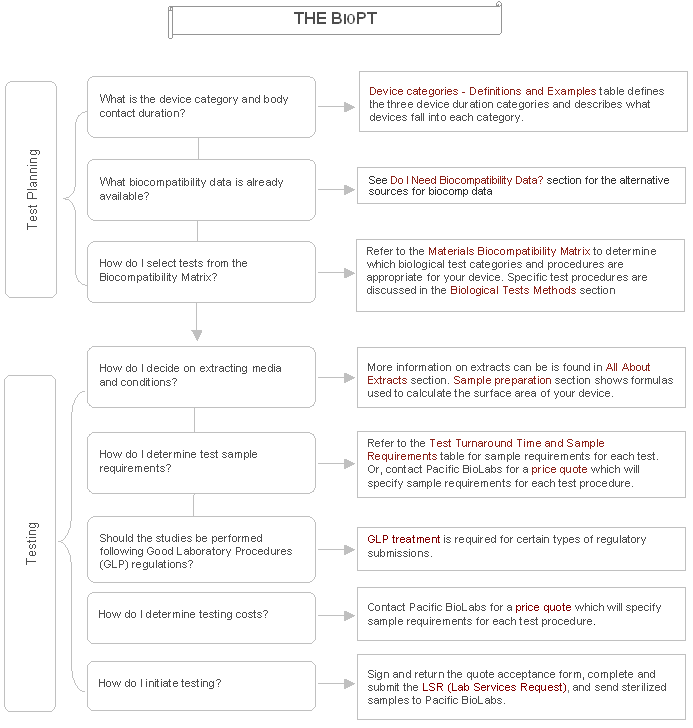
The chart below gives you an overview of the process. Follow the links to get more detail on each specific topic. including information on materials characterization & analytical testing of devices.
Extraction Media for Biocompatibility Testing
Medical device biocompatibility problems are most often caused by toxins that leach out of the device into the surrounding tissues or body fluids. So in the laboratory, extracts of device materials are often used in assessing biocompatibility. These extracts are generally prepared using exaggerated conditions of time and temperature to allow a margin of safety over normal physiological conditions.
Analytical extraction studies allow the chemist to identify and quantitate specific leachable moieties. This data can in turn help the device toxicologist or risk assessor determine the worst case scenario for patient exposure and the risk to patient health.
Extracts are also used in many of the biological tests specified by ISO 10993. Table 1 lists the most commonly used extracting media. For most devices, only saline and vegetable oil extracts are needed.
Extracts are selected on the basis of the biological environment in which the test material is to be used. A saline (SCI) extract approximates the aqueous, hydrophilic fluids in the body. It also permits the use of extreme temperatures in preparing the extracts, thus simulating certain sterilization conditions.
Tissue culture media may even more closely approximate aqueous body fluids, but cannot be used for high temperature extractions. Vegetable oils are non-polar, hydrophobic solvents and simulate the lipid fluids in the body. For technical reasons, DMSO extracts are often used in certain genotoxicity and sensitization tests. Two other common extracting media – Alcohol in SCI and PEG – should be used only if they approximate the solvent properties of drugs or other materials that will contact the device during its normal use.
Extraction conditions (temperature and time) should be at least as extreme as any conditions the device or material will encounter during sterilization or clinical use. Generally, you will want to choose the highest extraction temperature that does not melt or fuse the material or cause chemical changes. To provide some margin of safety for use conditions, Pacific BioLabs recommends an extraction condition of at least 50°C for 72 hours. For devices that are susceptible to heat, an extraction condition of 37°C for 72 hours may be acceptable. Table 2 lists common extraction conditions.
|
|
Sample Preparation
The simplest method for determining the surface area of a device is usually to use the CAD program from the design engineering group. Typically the surface area can be calculated with a just a few keystrokes. Alternatively, you can calculate the surface area using the equations below. Or you can submit a sample device and/or an engineering drawing to Pacific BioLabs, and our staff will perform the calculations.
Typically, the standard surface area of your device is used to determine the volume of extract needed for each test performed. This area includes the combined area of both sides of the device but excludes indeterminate surface irregularities. If the surface area cannot be determined due to the configuration of the device, a mass/volume of extracting fluid can be used. In either case, the device is cut into small pieces before extraction to enhance exposure to the extracting media. In some cases, it is not appropriate to cut the device; such devices are tested intact.
The Test Turnaround Time and Sample Requirements table lists the amount of sample required for many procedures. Generally, we recommend using the ratio of sample to extracting media specified in ISO 19993-12 (i.e. either 6 cm²/mL or 3 cm²/mL, depending on the thickness of the test material). For some types of materials, the ratio used for USP Elastomeric Closures for Injections (1.25 cm² per mL) is preferred.
Formulas For Surface Area Calculation
|
|
Legend
| A = surface area | ID = inner diameter> | |
| OD = outer diameter | L = length | |
| W = width | R = radius | |
| RR = ring radius (circular ring) | rc = cross section radius (circular ring) | |
| X, Y = longest and shortest distances through the center of an ellipse | π = 3.14 | |
| h = height | b = base length | |
| p, q = length of the parallel sides of a trapezoid | n = number of sides of a polygon | |
| r0 = ½ OD | ri = ½ ID |
ISO 10993 – Biological Evaluation Of Medical Devices: Listing Of Individual Parts
| |||||||||||||||||||||||||||||||||||||||||||
* = The United States ISO Member Body, ANSI/AAMI, is considering a version of this document for use in the U.S.
Device Categories – Definitions & Examples
| |||||||||||||||||||||||||
ISO Materials Biocompatibility Matrix
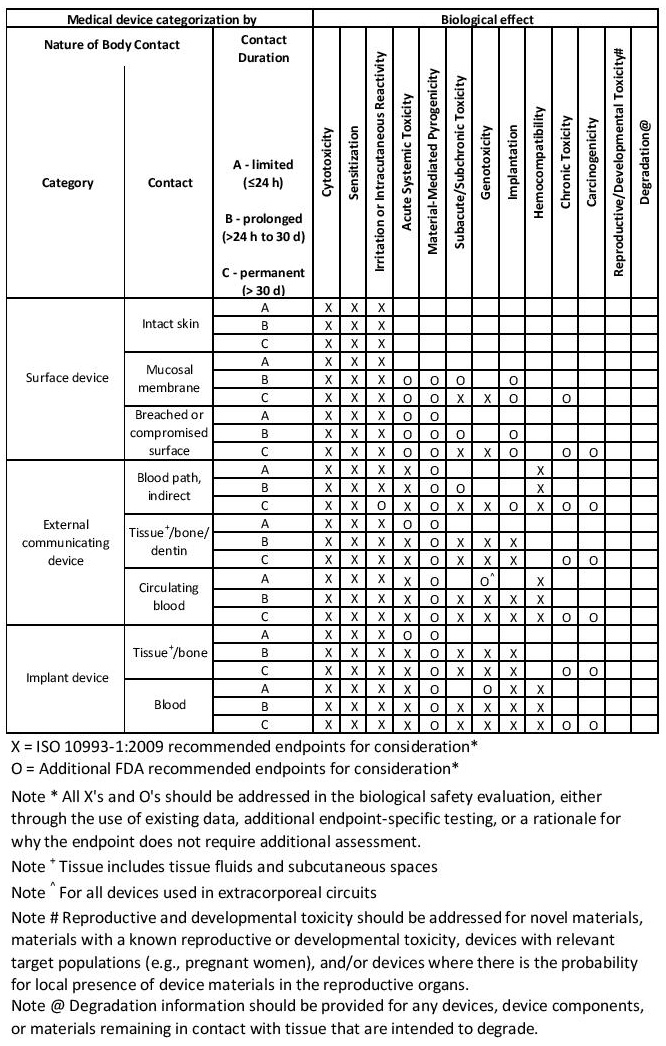
| This table is only a framework for the development of an assessment program for your device and is not a checklist. | ||
| Consult with the FDA before performing any biocompatibility testing if you are submitting an IDE or you have a device/drug combination. | ||
For current test turnaround times and sample amount requirements download printable version of our booklet Assessing Biocompatibility – A Guide for Medical Device Manufacturers(PDF).